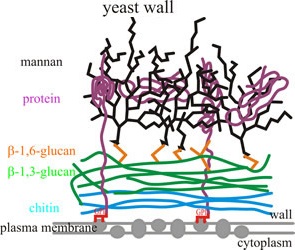
In a context moving towards antibiotic-free livestock production, research in feed additives has largely increased to evaluate different products and their effects on animal health and immune response, in addition to animal productivity [Xue at al., 2017]. Among the different products, prebiotics such as yeast cell wall (YCW) extracts have been widely used; they comprise mannanoligosaccharides (MOS), mannan-proteins, β (1,3)- glucans, β (1,6)-glucans, chitin, and glycophospholipid surface proteins associated with the plasma membrane [Guang-Da et al., 2017].
Background
The different yeast fractions can modulate animal gut health by different mechanisms. Dietary supplementation with MOS has been found to improve gut morphology in terms of longer villi, shorter crypts, and a higher number of goblet cells [Baurhoo et al., 2007]. In infected chicks (Salmonella typhimurium and Clostridium perfringens), MOS positively affected immune and metabolic pathways in the gut and reduced its colonization [Caly et al., 2015; Faber et al., 2012; Hashim et al., 2018].
In broiler chickens, dietary supplementation with YCW extracts containing different rates of MOS and β-glucans might improve growth performance and gut morphology [Muthusamy et al., 2011; Santin et al., 2001]
the present study aimed at evaluating the effect of dietary supplementation with YCW extracts (mainly mannanoligosaccharides and β-glucans) from Saccharomyces cerevisiae (SafMannan®, Phileo, Lesaffre, Marcq-en-Baroule Cedex, France) on growth performance and slaughter results, health, gut morphology, immune status and gut transcriptome in broiler chickens.
Methods
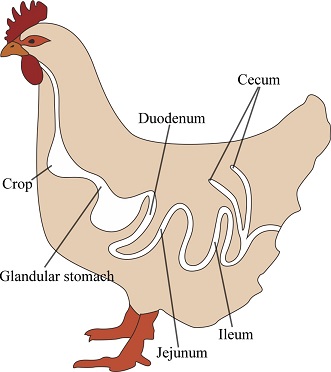
The trial was performed at the poultry house of the Experimental Farm of the University of Padova (Legnaro, Padova, Italy). Chicks were individually weighed on their arrival, identified by a leg ring, and weighed for live weight once a week until slaughtering at 44 d. Pen feed consumption was measured daily during the trial.
For each treatment, three commercial diets in crumble form were administered during the trial as usual, i.e. from 1 to 14 d; from 15 to 28 d; and from 29 d until slaughtering (on d 44). All diets were formulated to satisfy broiler nutritional requirements.
The treatment diets contained different levels of Safmannan® (mannan-oligosaccharides > 20%; β-glucans 1,3 and 1,6 > 20%) as source of YCW extracts (250, 500, and 250 mg/kg in the three diets, respectively) as used in the field.
Results
1-Dietary YCW supplementation did not affect final live weight, whereas it decreased feed intake (114 to 111 g/d; P ≤ 0.10) and improved feed conversion (1.74 to 1.70; P ≤ 0.01).
2-Regarding the gut, YCW supplementation tended to increase villi height (P = 0.07); it also increased the number of goblet cells and reduced the density of CD45+ cells compared to diet C (P < 0.001). The increase in the number of goblet cells can be positively considered in view of the protective effect of mucus production by goblet cells under any challenging conditions
3- Our study showed differences in the mucosal immune responses as the density of intraepithelial leukocytes (especially CD45+) was lower when chickens received a diet supplemented with yeast cell walls. A lower immune reaction in these birds could mean that YCW protected animals from any agent/cause that could have challenged animals during the trial, which is consistent with the increased number of goblet cells, and thus increased mucin production, in the gut of these animals.
4- In the gut transcriptome, four genes were expressed more in broilers fed diet Y compared to diet C, i.e. cytochrome P450, family 2, subfamily C, polypeptide 23b (CYP2C23B), tetratricopeptide repeat domain 9 (TTC9), basic helix-loop-helix family member e41 (BHLHE41), and the metalloreductase STEAP4. Only one gene set (HES_PATHWAY) was significantly enriched among the transcripts more expressed in broilers fed diet Y. Notably, several enriched gene sets are implicated in immune functions and related to NF-κB signaling, apoptosis, and interferon signals.
5- The GSEA analysis demonstrates that immune, pro-inflammatory and pro-apoptotic processes are activated to a higher extent in chicks fed the control diet compared to those fed with the supplemented diet. The activation of these pathways might be a consequence of higher stress levels due to any challenging condition (e.g. nutritional challenge, pathogens infection, inflammatory processes) in control animals, and is in agreement with the higher density of intraepithelial leukocytes and the reduced amount of goblet cells observed in this experimental group.
Overall
based on the biological functions of the DEGs and the significantly enriched gene sets, we hypothesize that molecular pathways mostly impacted by YCW extract supplementation are those related to immunity. This suggests that yeast supplementation might play a key role in triggering anti-inflammatory mechanisms and an animal’s response to pathogens.
References
[1]-Baurhoo, B., Phillip, L. and Ruiz-Feria, C.A., 2007. Effects of purified lignin and mannan oligosaccharides on intestinal integrity and microbial populations in the ceca and litter of broiler chickens. Poultry science, 86(6), pp.1070-1078.
[2]-Caly, D.L., D’Inca, R., Auclair, E. and Drider, D., 2015. Alternatives to antibiotics to prevent necrotic enteritis in broiler chickens: a microbiologist’s perspective. Frontiers in microbiology, 6, p.1336.
[3]-Faber, T.A., Dilger, R.N., Iakiviak, M., Hopkins, A.C., Price, N.P. and Fahey Jr, G.C., 2012. Ingestion of a novel galactoglucomannan oligosaccharide-arabinoxylan (GGMO-AX) complex affected growth performance and fermentative and immunological characteristics of broiler chicks challenged with Salmonella typhimurium. Poultry science, 91(9), pp.2241-2254.
[4]-Hashim, M.M., Arsenault, R.J., Byrd, J.A., Kogut, M.H., Al-Ajeeli, M. and Bailey, C.A., 2018. Influence of different yeast cell wall preparations and their components on performance and immune and metabolic pathways in Clostridium perfringens-challenged broiler chicks. Poultry science, 97(1), pp.203-210.
[5]-Muthusamy, N., Haldar, S., Ghosh, T.K. and Bedford, M.R., 2011. Effects of hydrolysed Saccharomyces cerevisiae yeast and yeast cell wall components on live performance, intestinal histo-morphology and humoral immune response of broilers. British poultry science, 52(6), pp.694-703.
[6]-Santin, E., Maiorka, A., Macari, M., Grecco, M., Sanchez, J.C., Okada, T.M. and Myasaka, A.M., 2001. Performance and intestinal mucosa development of broiler chickens fed diets containing Saccharomyces cerevisiae cell wall. Journal of Applied Poultry Research, 10(3), pp.236-244.
[7]-Xue, G.D., Wu, S.B., Choct, M. and Swick, R.A., 2017. Effects of yeast cell wall on growth performance, immune responses and intestinal short chain fatty acid concentrations of broilers in an experimental necrotic enteritis model. Animal Nutrition, 3(4), pp.399-405.

 Fa
Fa En
En