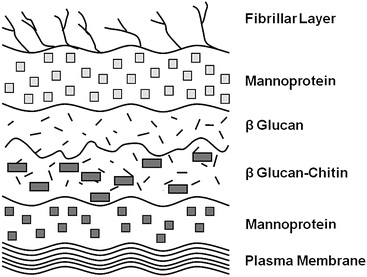
Yeast cell walls (YCW) contain mannoproteins, beta (1,3)-glucans, beta (1,6)-glucans, chitin and glycophospholipid surface proteins associated with the plasma membrane. The YCW is known to have prebiotic properties with efficacy for modulating immunity and gut microflora (Pourabedin and Zhao, 2015). As such, YCW is a potential replacement for dietary sub-therapeutic antibiotic. Prebiotics derived from YCW have been found to promote the production of immunoglobulin (Ig) (Czech et al., 2010) that plays as an important part in host immunity.
By recognizing and binding particular antigens, Ig controls the prevalence and severity of infections (Goudswaard et al., 1977; Desmidt et al., 1998). Prebiotics derived from YCW were also found to prevent the disease by inducing the pro-inflammatory responses (Monsan and Paul, 2008) as inflammation mediates the host immunity to against the disease such as acute bacterial infection (Medzhitov, 2007). The mode of action of YCW also involves altering gut microflora composition via competitive exclusion (Callaway et al., 2008), production of antimicrobial agents (Chen et al., 2007; Munoz et al., 2012) and changing the fermentation pattern of the gut microflora (Donalson et al., 2008).
yeast cell wall and subclinical necrotic enteritis
The effect of YCW during subclinical NE has not been investigated. The present study was designed to examine the role of YCW in performance, intestinal NE lesions, pro- and anti-inflammatory markers, Ig production and intestinal metabolite profile in the broiler chickens challenged by subclinical NE.
Materials and methods
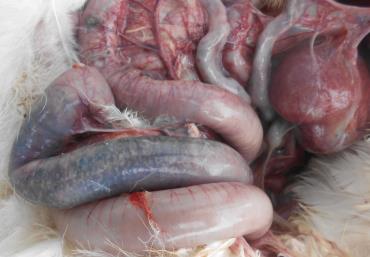
This study was conducted at University of New England, Armidale, New South Wales, Australia. The study employed a 2 × 3 factorial arrangement of treatments with 8 replicate pens per treatment and 15 birds per pen. Factors were: NE challenge (no or yes), and additive :
1- (none [control]
2- YCW [Actigen, Alltech, USA; 800, 400, 200 g/t; starter, grower and finisher, respectively], or antibiotics [AB].
The AB treatment consisted of a combination of Zn bacitracin and salinomycin. The diets were based on wheat, soybean meal, sorghum, canola meal, meat and bone meal and formulated to meet Ross 308 nutrient specifications.
Results
Challenged birds had higher feed conversion ratio (FCR) than unchallenged birds on d 35 (P < 0.05). Dietary inclusion of antibiotics decreased FCR regardless of challenge (P < 0.05) on d 24 and 35. Inclusion of YCW reduced serum interleukin-1 (IL-1) concentration in NE challenged birds (P < 0.01) and increased immunoglobulin (Ig) G (P < 0.05) and Ig M (P < 0.05) levels compared to other dietary treatments regardless of challenge.
Perhaps YCW does not affect the colonization of Clostridium perfringens on the intestinal epithelium directly, i.e., lesion development, but rather may have other roles, for example, immunity of the birds, that determine different performance responses in clinical or subclinical NE outbreaks.
Immunomodulation as a result of dietary YCW has been postulated and the current study showed YCW exhibited antiinflammatory effects and promoted Ig production. This implies that the cost of NE consists of 2 parts e a dysfunctional intestine and the cost of immunity respectively. Yeast cell wall extract has also been reported to terminate systemic inflammation earlier than virginiamycin in the findings of Baurhoo et al. (2012). It is likely that YCWcompetitively excludes pathogens whose cell integrity will be maintained whereas the use of antibiotics results in bacterial fragmentation during cytolysis (Yamasaki et al., 2009).
This would lead to release of cellular contents, including microbial DNA and prototypical enterotoxins (Su et al., 2006) thus inducing inflammatory response. On the other hand, in this study YCW promoted Ig secretion to be suggested that might offer protection against the infection (Cetin et al., 2005; Czech et al., 2010). Moreover, YCW increased butyric acid and formic acid production in the ceca. To some extent, SCFA control bacterial infections, act as potential antibiotic replacers, and benefit the gut health as an energy source for enterocytes (Hernandez et al., 2006; García et al., 2007; Fernandez-Rubio et al., 2009).
Conclusion
Supplementation of YCW may offer protection against subclinical NE due to its effects on antinflammation, immunoglobulin secretion and SCFA production. However, continuous feeding of YCW, especially after birds have recovered from subclinical NE, may diminish the positive effect of YCW on performance. The current study showed the range and regime of YCW that may be deployed under antibiotic-free production situations.
References
Baurhoo B, Ferket P, Ashwell CM, de Oliviera J, Zhao X. Cell walls of Saccharomyces cerevisiae differentially modulated innate immunity and glucose metabolism during late systemic inflammation. PLoS One 2012;7, e30323.
Callaway TR, Edrington TS, Anderson RC, Harvey RB, Genovese KJ, Kennedy CN, et al. Probiotics, prebiotics and competitive exclusion for prophylaxis against bacterial disease. Anim Health Res Rev 2008;9:217e25.
Cetin N, Güçlü BK, Cetin E. The effects of probiotic and mannanoligosaccharide on some haematological and immunological parameters in turkeys. Transbound Emerg Dis 2005;52:263e7.
Chen YS, Srionnual S, Onda T, Yanagida F. Effects of prebiotic oligosaccharides and trehalose on growth and production of bacteriocins by lactic acid bacteria. Lett Appl Microbiol 2007;45:190e3.
Czech A, Grela ER, Mokrzycka A, Pejsak Z. Efficacy of mannanoligosaccharides additive to sows diets on colostrum, blood immunoglobulin content and production parameters of piglets. Pol J Vet Sci 2010;13:525e31.
Desmidt M, Ducatelle R, Mast J, Goddeeris BM, Kaspers B, Haesebrouck F. Role of the humoral immune system in Salmonella enteritidis phage type four infection in chickens. Vet Immunol Immunopathol 1998;63:355e67.
Donalson LM, Kim WK, Chalova VI, Herrera P, McReynolds JL, Gotcheva VG, et al. In vitro fermentation response of laying hen cecal bacteria to combinations of fructooligosaccharide prebiotics with alfalfa or a layer ration. Poult Sci 2008;87: 1263e75.
Fernandez-Rubio C, Ordonez C, Abad-Gonzalez J, Garcia-Gallego A, Pilar Honrubia M, Jose Mallo J, et al. Butyric acid-based feed additives help protect broiler chickens from Salmonella Enteritidis infection. Poult Sci 2009;88: 943e8.

 Fa
Fa En
En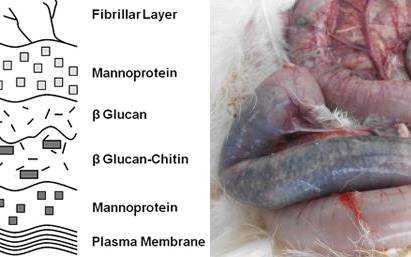
بدون دیدگاه